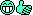Why did a bottle of water freeze...
Discussion
After I took it out of the icebox?
I put a 500ml plastic bottle of water in the icebox of the fridge at about 4pm when I took it out at 6pm it was cold but still liquid.
The top was stiff to undo but when I undid the top ice formed at the top and rapidly made its way to the bottom leaving the bottle completely frozen solid.
I put a 500ml plastic bottle of water in the icebox of the fridge at about 4pm when I took it out at 6pm it was cold but still liquid.
The top was stiff to undo but when I undid the top ice formed at the top and rapidly made its way to the bottom leaving the bottle completely frozen solid.
I had this happen to me once with a bottle of wine...just wish I had videoed it.
I'd left it in the freezer too long and went to get it...it was still liquid .. and had a screw cap.
I opened the cap and started to pour into a glass and it looked like the freezing event started in the bottom of the glass and worked it's way back up the poured wine into the bottle...completely bizarre & left me with a "wine slush" in the glass and a "spout" of frozen wine + frozen wine in the bottle.
I'd left it in the freezer too long and went to get it...it was still liquid .. and had a screw cap.
I opened the cap and started to pour into a glass and it looked like the freezing event started in the bottom of the glass and worked it's way back up the poured wine into the bottle...completely bizarre & left me with a "wine slush" in the glass and a "spout" of frozen wine + frozen wine in the bottle.
Strangely I was sent this the other day, haven't tried it yet...
http://overheard.liketodiscover.com/instant-adult-...
http://overheard.liketodiscover.com/instant-adult-...
Lynchie999 said:
I suppose its the lowering of pressure .. which then lowers the temperature ... ??
There is no lowering of pressure. The freezing temperature is unchanged, as evidenced by the water freezing when tapped. What is happening is that there is no seed crystal for the ice, and nothing to form one around (like a speck of dust or a scratch in the container), so there are no ice crystals. When you tap the water you jostle the molecules and a crystal forms. Alternatively if the water is cooled enough a crystal will form spontaneously.Ice nucleators are very important in the formation of crystals, but pressure may also play a part. As the water in the bottle cools it contracts until it reaches 4.0 degrees, after which it starts to expand again. So at 0 degrees the pressure inside the bottle is higher than it was at 4.0. As the phase diagram shows, increasing pressure depresses the freezing point. Removing the cap will release the pressure and may trigger ice formation. In the Antarctic there is a phenomenon know as anchor ice. Anchor ice forms on solid surfaces under the water, but only down to about 10m. Beyond that the higher pressure prevents its formation. So too with some Antarctic fish. They happily live in deep water at high pressure, but will freeze solid if they are caught and brought to shallower depths.
The pressure differences in a plastic bottle from changes in temperature of liquid water are tiny, not enough to affect the melting point significantly. The volume difference between water at 30C and 4C is only 0.43% which in a typical plastic bottle would have negligible effect on the pressure.
Also:
The melting point of ice falls by 0.0072 °C for each additional atm of pressure applied.
From http://en.wikipedia.org/wiki/Regelation
A typical plastic bottle can hold about 10-12 atm before bursting so the maximum reduction in melting point for ice you could manage without bursting the bottle is 0.09C, which would be undetectable in a domestic setting.
It's really not about pressure, it's all down to nucleation, which makes sense as banging the bottle doesn't affect the internal pressure, other than giving rise to a short pressure spike from the shock (which under the pressure hypothesis should melt the ice, not cause it to freeze).
Also:
The melting point of ice falls by 0.0072 °C for each additional atm of pressure applied.
From http://en.wikipedia.org/wiki/Regelation
A typical plastic bottle can hold about 10-12 atm before bursting so the maximum reduction in melting point for ice you could manage without bursting the bottle is 0.09C, which would be undetectable in a domestic setting.
It's really not about pressure, it's all down to nucleation, which makes sense as banging the bottle doesn't affect the internal pressure, other than giving rise to a short pressure spike from the shock (which under the pressure hypothesis should melt the ice, not cause it to freeze).
Flibble said:
The volume difference between water at 30C and 4C is only 0.43% which in a typical plastic bottle would have negligible effect on the pressure.
Agreed, but the difference in volume from 4 degrees to 0 degrees is a little over 9%. This would have an influence on pressure.CR6ZZ said:
Agreed, but the difference in volume from 4 degrees to 0 degrees is a little over 9%. This would have an influence on pressure.

The volume changes by around 0.01% between 4C and 0C. The density of water below 0C isn't much higher than at 4C in fact, at -10C it's approximately as dense as at 20C, so minimal difference.
Obviously once it freezes the density of ice is much lower, but it has to actually freeze for that, and the whole discussion is about it not freezing, hence no significant volume change.
Also as noted, the increase in pressure would be too small to have a noticeable effect on the freezing point of water since any pressure that did have such an effect would burst the bottle.
None of these bottles are completely 100% filled with water though. There is always some air trapped at the top.
The volume of this trapped air in the bottle would reduce with the drop in temp. Depending on the ratio of water to air inside the bottle the pressure may actually reduce despite any increase in liquid volume, as evidenced by a partially-filled bottle sealed at room temp contracting when cooled in a fridge.
The volume of this trapped air in the bottle would reduce with the drop in temp. Depending on the ratio of water to air inside the bottle the pressure may actually reduce despite any increase in liquid volume, as evidenced by a partially-filled bottle sealed at room temp contracting when cooled in a fridge.
thegreenhell said:
None of these bottles are completely 100% filled with water though. There is always some air trapped at the top.
The volume of this trapped air in the bottle would reduce with the drop in temp. Depending on the ratio of water to air inside the bottle the pressure may actually reduce despite any increase in liquid volume, as evidenced by a partially-filled bottle sealed at room temp contracting when cooled in a fridge.
This is true, however given that bottles contract under those circumstances the volume change from that limits any pressure change. Also the melting point of ice isn't much affected by reduced pressure (until you get to the triple point).The volume of this trapped air in the bottle would reduce with the drop in temp. Depending on the ratio of water to air inside the bottle the pressure may actually reduce despite any increase in liquid volume, as evidenced by a partially-filled bottle sealed at room temp contracting when cooled in a fridge.
Some of the examples previously given in this thread involved glass bottles, which would see a pressure drop inside.
However, I brought up the air pressure factor as a counterpoint to those discussing the minutiae of liquid pressure increases inside the bottle, and I think pressure is a red herring in this phenomenon anyway.
However, I brought up the air pressure factor as a counterpoint to those discussing the minutiae of liquid pressure increases inside the bottle, and I think pressure is a red herring in this phenomenon anyway.
Gassing Station | Science! | Top of Page | What's New | My Stuff